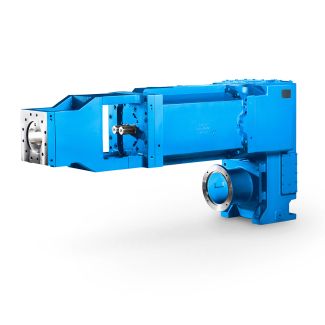
Bevel-helical gear reducers B4 cle No LP Z Sealing singleside high spee B4DH-12-C
In stock
SKU
B4DH-12-C
$37,500.00
Flender/Flender Gear Units/Bevel-helical gear reducers B4
ture and density) on the composition was determined by observing the critical opale- scence. Both the boiling pressure and the critical state variables are expressed by simple, empirical correlations with an accuracywhich hard1 exceeds the experimental error limit, if at
critical state variables are expressed by simple, empirical correlations with an accuracywhich hard1 exceeds the experimental error limit, if at  all. The experimental data can also be describecfwith virtually thesame accuracy using simple equations of state of the van der
all. The experimental data can also be describecfwith virtually thesame accuracy using simple equations of state of the van der  Waals type. These equations permit calculation of the phase equilibrium over the entire concentration range and in the entire temperature
Waals type. These equations permit calculation of the phase equilibrium over the entire concentration range and in the entire temperature  range of interest for refrigeration applications. The R1a/R1a system displays negative deviation from,Raoult' law, becoming more pronounced at lower temperatures. As regards the pressure situation in compression refrigerating machine, the va our pressure curve of an equimolar mixture is thus slightly more favourable than that orthe pure substances. Even with an equimolar mixture, the concentration difference between vapour and liquid is no greater than approx. 2 mole per cent in the temperature range between -4 'Cand +4 '.Thus,for refrigeration applica- tions, mixture of this kind can be treated in very much the same way as pure substance. The real mixing behaviour of gaseous mixtures of R1a and R1a is characterised by negative values for the excess volume in the low density range. It can thus be concluded that the attractive interaction in the gas phase is also stronger in the mixture than in the pure substances. Viewed in relative terms, the deviation from ideal miscibility is not very great, but it is nevertheless in the percent range durin the suction cycle of compression refrigerating machine. As the negative excess volume8as favourable effect on the speci- fic suction volume of the compressor, it can be expected that use of an equimolar mixture of R1a and R1a will even yield somewhat better energy consumption data than the pure substances. ,. , 2 Thus, neither the thermodynamlc properties nor the ther
range of interest for refrigeration applications. The R1a/R1a system displays negative deviation from,Raoult' law, becoming more pronounced at lower temperatures. As regards the pressure situation in compression refrigerating machine, the va our pressure curve of an equimolar mixture is thus slightly more favourable than that orthe pure substances. Even with an equimolar mixture, the concentration difference between vapour and liquid is no greater than approx. 2 mole per cent in the temperature range between -4 'Cand +4 '.Thus,for refrigeration applica- tions, mixture of this kind can be treated in very much the same way as pure substance. The real mixing behaviour of gaseous mixtures of R1a and R1a is characterised by negative values for the excess volume in the low density range. It can thus be concluded that the attractive interaction in the gas phase is also stronger in the mixture than in the pure substances. Viewed in relative terms, the deviation from ideal miscibility is not very great, but it is nevertheless in the percent range durin the suction cycle of compression refrigerating machine. As the negative excess volume8as favourable effect on the speci- fic suction volume of the compressor, it can be expected that use of an equimolar mixture of R1a and R1a will even yield somewhat better energy consumption data than the pure substances. ,. , 2 Thus, neither the thermodynamlc properties nor the ther| Model Type | Bevel-helical gear reducers B4 |
|---|---|
| Gear Type | Bevel Helical Gear |
| Weight (kg) | 1750.000000 |
| Ratio Range | 1 : 100…400 |
| Low Speed Output | Hollow shaft with shrink disk |
| Nominal Torque | 78000 Nm |
| Mounting Arrangements | Horizontal mounting position |
| Manufacturer | WALTHER FLENDER GMBH |
| Country of Manufacture | Germany |
| Data Sheet & Drawings | Bevel-helical gear reducers B4 cle No LP Z Sealing singleside high spee B4DH-12-C |